Bankim Nagar, Siliguri, West Bengal
- GST NO. : 19BHDPS7640K1ZR
Top Challenges When Choosing the Best EEG Equipment for Accurate Diagnosis
In recent years, the demand for high-quality EEG equipment has surged, driven by advancements in neurology and the rising prevalence of neurological disorders. According to a report by Grand View Research, the global EEG devices market size was valued at approximately $1.6 billion in 2020 and is anticipated to expand at a CAGR of 7.8% from 2021 to 2028. As healthcare professionals aim for more accurate diagnoses, selecting the right EEG equipment has become a critical challenge. With various options flooding the market, it can be overwhelming to identify a reliable supplier who meets stringent quality standards. This blog aims to navigate these challenges by outlining key considerations for healthcare providers looking to procure high-quality EEG equipment, ensuring that they can make informed decisions in their pursuit of accurate diagnosis and effective patient care.
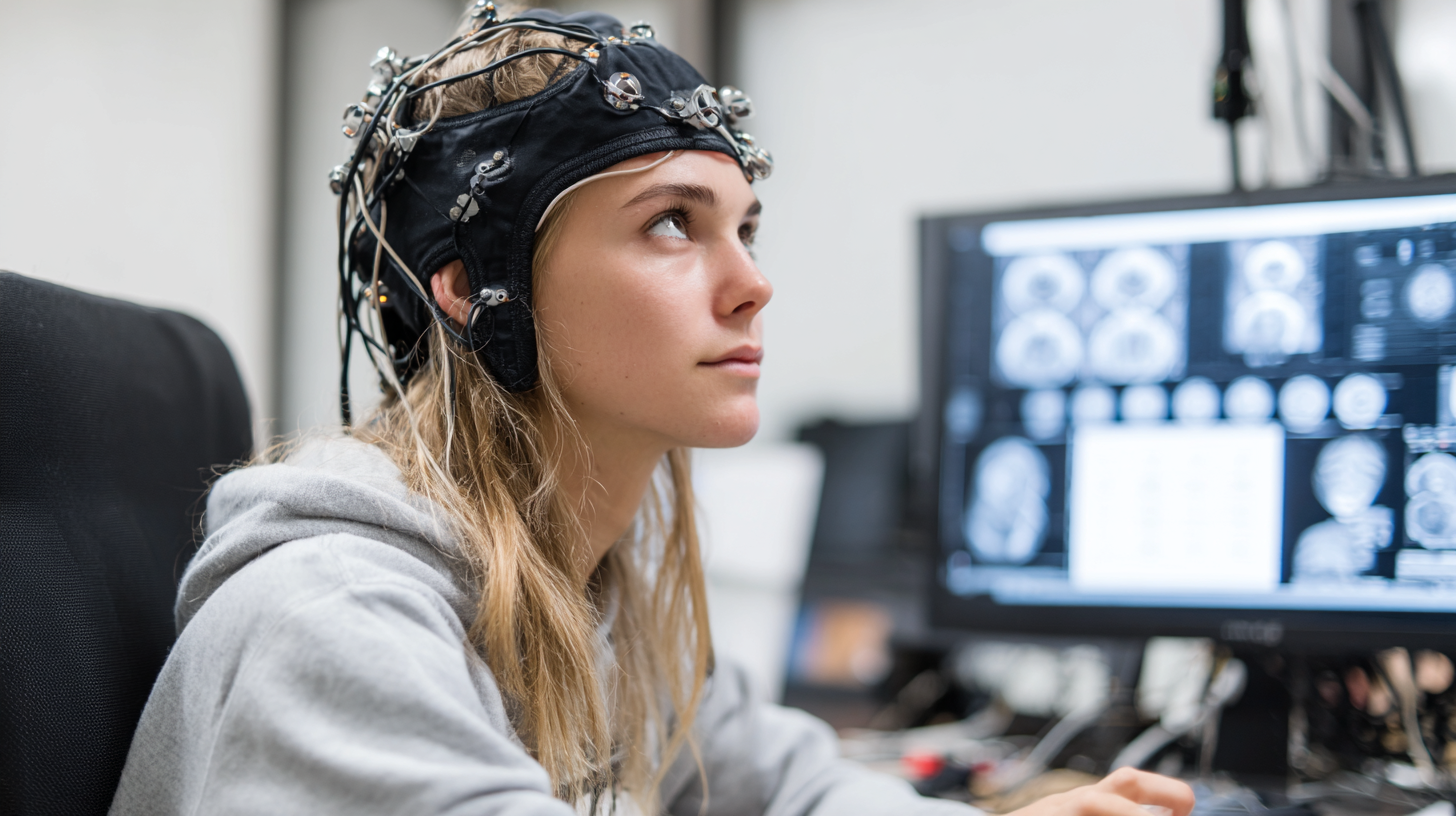
Key Features to Consider When Selecting EEG Equipment for Diagnoses
When selecting EEG equipment for accurate diagnoses, several key features must be considered to ensure optimal performance and reliability. One of the primary elements is the number of channels; studies indicate that EEG systems with 32 or more channels provide enhanced spatial resolution and improve the detection of brain abnormalities. According to a report by the International Society for Neurofeedback and Research, a higher channel count correlates with better diagnostic accuracy in various neurological conditions, including epilepsy and sleep disorders.
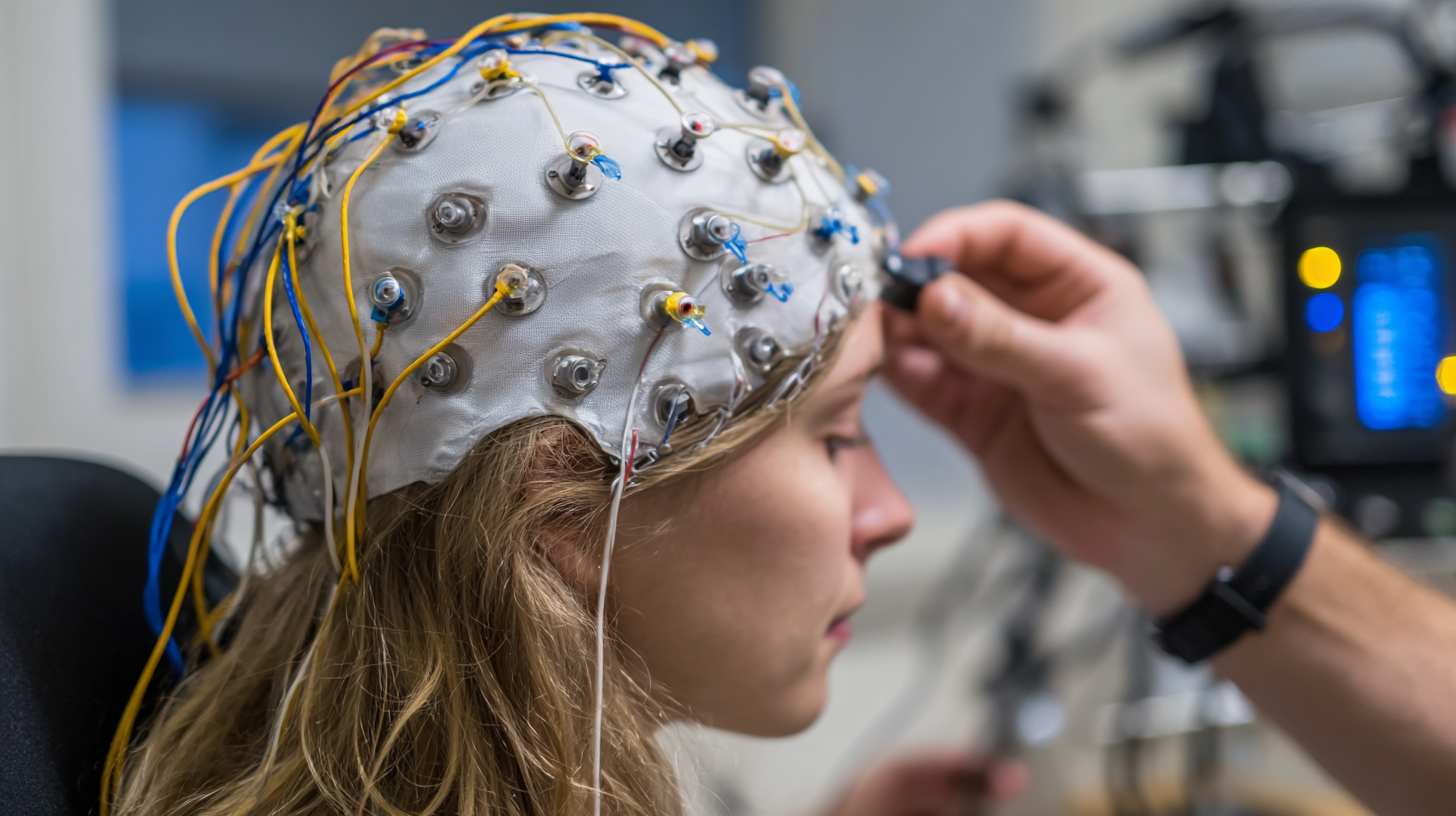
Another critical aspect is the quality of data acquisition and the presence of advanced filtering options. High-performance EEG systems that utilize digital signal processing can significantly reduce noise and artifacts, providing cleaner data for analysis. A comprehensive market analysis by Grand View Research highlights that EEG systems with integrated software for real-time analysis and patient monitoring are becoming increasingly valuable in clinical settings. This capability not only aids in prompt diagnosis but also enhances the overall efficiency of patient care. Thus, evaluating these features is essential for clinicians aiming to invest in the best EEG equipment for precise and effective diagnoses.
Understanding the Variability in EEG Equipment Quality Across Manufacturers
When selecting EEG equipment for accurate diagnosis, it’s crucial to recognize that not all devices are created equal. Understanding the variability in EEG equipment quality across manufacturers can significantly impact the reliability of diagnoses. Each manufacturer employs different technologies and methodologies, leading to discrepancies in the sensitivity, specificity, and overall performance of their devices. Therefore, thorough research and comparison are essential to determine which equipment meets both clinical needs and quality standards.
One essential tip is to consider the reputation of the manufacturer. Look for companies that have a proven track record in the field of neurology and customer satisfaction. User reviews and testimonials can provide valuable insights into the equipment's performance and reliability. Additionally, check if the equipment complies with international safety and quality standards, as this can be indicative of its durability and effectiveness in clinical settings.
Another tip is to prioritize compatibility with existing systems. If your facility already utilizes specific software or hardware, ensure that the new EEG equipment can integrate seamlessly with your current setup. This will not only enhance workflow efficiency but also streamline data management and analysis, ensuring that you get the most out of your diagnostic efforts.
The Importance of Calibration and Maintenance in EEG Diagnostics
When selecting the best EEG equipment for accurate diagnosis, the importance of calibration and maintenance cannot be overstated. According to a study published in the Journal of Clinical Neurophysiology, improper calibration can lead to diagnostic inaccuracies as high as 30%, highlighting the critical need for regular calibration protocols. Besides ensuring the accuracy of readings, proper calibration helps maintain the integrity of data over time, allowing healthcare providers to make informed decisions based on reliable EEG results.
Maintenance is equally crucial, as EEG equipment is subject to wear and tear from frequent use. A report from the International Society for Neurofeedback and Research emphasizes that routine maintenance can significantly extend the lifespan of EEG devices and reduce the likelihood of unexpected failures during critical diagnostic procedures. Facilities that implement a robust maintenance schedule report up to a 50% reduction in equipment downtime, ensuring that diagnostic services remain uninterrupted. Investing in these practices not only enhances the effectiveness of EEG diagnostics but also ultimately leads to better patient outcomes.
Navigating Regulatory Standards for EEG Equipment in Global Markets
When selecting EEG equipment for accurate diagnostic purposes, understanding the regulatory standards across global markets is paramount. Each country has established specific guidelines and requirements that manufacturers must adhere to before their products can reach the market. Navigating these regulatory landscapes can be daunting, especially when considering that standards can vary significantly from one region to another. For instance, the FDA in the United States has stringent approval processes, while CE marking in Europe ensures compliance with health, safety, and environmental protection standards. This necessitates that manufacturers not only invest in the quality and efficacy of their devices but also remain well-informed about the varying regulatory obligations.
Moreover, the implications of non-compliance are serious. Failure to meet regulatory requirements can lead to costly delays in product launches, recalls, or even legal penalties. Therefore, manufacturers should engage in thorough market research and maintain open communication with regulatory bodies to ensure their EEG equipment aligns with local standards. Understanding these regulations is not just about compliance; it is crucial for building trust with healthcare providers and patients alike, as it directly influences the credibility and reliability of the diagnostic tools being offered. By prioritizing regulatory adherence from the outset, companies can facilitate smoother entry into global markets and contribute to better patient outcomes.

Cost vs. Quality: Balancing Budget Constraints with Diagnostic Accuracy
When selecting EEG equipment, one of the foremost considerations is the balance between cost and quality. A 2021 report from the International Society of Electroencephalography highlights that while high-end EEG devices can exceed $100,000, many clinics are pressured to maximize their diagnostic capabilities within tight budgets. This creates a dilemma: investing in cutting-edge technology for improved accuracy or opting for more affordable options that may compromise diagnostic precision.
According to a study published in the Journal of Clinical Neurophysiology, lower-cost EEG systems often yield a diagnostic accuracy rate of approximately 75%—a significant gap compared to the 95% accuracy offered by premium systems. This disparity can lead to misdiagnosis, potentially resulting in treatment delays and increased healthcare costs in the long term. Therefore, healthcare providers must carefully evaluate their financial constraints against the potential consequences of inaccurate diagnoses, ensuring they choose equipment that meets their clinical needs without overspending. Ultimately, striking the right balance between cost and diagnostic fidelity can enhance patient outcomes and optimize resource utilization within the healthcare system.
Top Challenges When Choosing the Best EEG Equipment for Accurate Diagnosis
| EEG Equipment Type | Cost ($) | Number of Channels | Portability (Yes/No) | Diagnostic Accuracy (% Sensitivity) |
|---|---|---|---|---|
| Standard EEG System | $10,000 | 32 | No | 85% |
| Portable EEG System | $8,000 | 16 | Yes | 80% |
| High-Density EEG System | $15,000 | 128 | No | 92% |
| Wireless EEG System | $12,000 | 32 | Yes | 88% |
| Clinical EEG Lab System | $20,000 | 64 | No | 90% |

